

A vital ingredient for diverse
industrial applications, Titanium Dioxide TiO2
is the most commonly used white pigment in the world.
Titanium, the ninth most common
element in the earths crust, is a metal commonly found in plants and animals.
Titanium naturally interacts with oxygen to form titanium oxides, commonly
found in ores, indigenous dusts, sands and soils.
Titanium Dioxide is most widely used
as White Pigment because of its brightness and very high refractive index, in
which it is surpassed only by a very few other materials.
Pure Titanium Dioxide is a fine,
white powder that provides a bright, white pigment.
Titanium Dioxide TiO2 has been used
for a century in a range of industrial applications and More than four
million tons are used annually.
When used as a pigment, it is called
Titanium White, Pigment White 6 (PW6) of CI 77891.
When used as a food coloring, it has
E number E171.
Titanium dioxide is produced in two
main forms.
The primary form, comprising over
98% of total production, is pigment grade titanium dioxide.
The pigmentary form makes use of
titanium dioxide’s excellent light-scattering properties in applications that
require white opacity and brightness.
The other form in which titanium
dioxide is produced is as an ultrafine (nano particle) product.
This form is selected when different properties,
such as transparency and maximum ultraviolet light absorption, are required,
such as in cosmetic sunscreens.
Titanium Dioxide TiO2 - Rutile
Titanium Dioxide Rutile is a
multi-purpose grade pigment recommended for use when a single pigment is
required to perform well in interior and exterior gloss and semi-gloss systems.
Though less resistant to weathering than other
grades, R-902+ provides an excellent balance of performance properties.
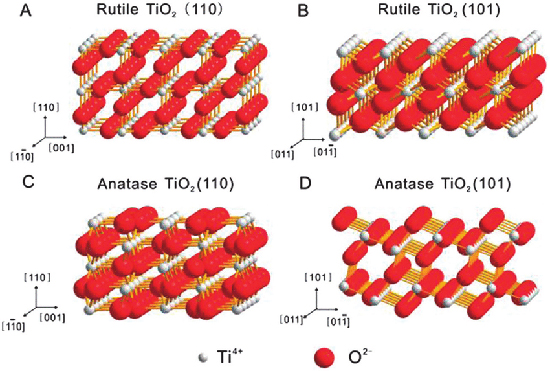
Anatase is one of the three mineral forms of
Titanium Dioxide TiO2.
A vital ingredient for diverse industrial applications,
Titanium Dioxide (TiO2) is the most commonly used white pigment in the world.
More than four million tonnes are used annually for a
variety of industrial applications like manufacturing of Paint, Rubber, Paper,
Detergents, Cosmetics, Printing Inks, Textiles, Plastics among other products.
Titanium Dioxide Anatase is always found as small, isolated
and sharply developed crystals, and like rutile, a more commonly occurring
modification of titanium dioxide, it crystallizes in the tetragonal system;
but, although the degree of symmetry is the same for both, there is no relation
between the interfacial angles of the two minerals, except in the prism-zone of
45° and 90°. The common pyramid of anatase,
parallel to the faces of which there are perfect cleavages, has an angle over
the polar edge of 82°9', the corresponding angle of rutile being 56°52½'.
It was on account of this steeper pyramid of
anatase that the mineral was named, by René Just Haüy in 1801, from the Greek
anatasis, “extension", the vertical axis of the crystals being longer than
in rutile.
There are also
important differences between the physical characters of anatase and rutile:
The former is less
hard (5.5–6 vs. 6-6.5 Mohs) and dense (specific gravity about 3.9 vs. 4.2).
Also, anatase is optically negative whereas rutile is positive, and its luster
is even more strongly adamantine or metallic-adamantine than that of rutile.




